FDA expedites approval of Cochlear’s Remote Check solution for cochlear implants
CENTENNIAL, Colo., April 7, 2020 /PRNewswire/ — Cochlear Limited (ASX: COH), the global leader in implantable hearing solutions, obtained U.S. Food and Drug Administration (FDA) approval on April 2 for its Remote Check solution. This approval is the first step in commercializing the product offering, which is anticipated by the end of 2020. Cochlear will immediately begin a controlled market release of Remote Check in the U.S. and Canada by partnering with hearing healthcare providers and hospitals to quickly reach those most in need of audiological care during the COVID-19 pandemic, which supports social distancing by providing an alternative to in-person appointments.
Remote Check is designed to be a convenient, at-home testing tool that allows Cochlear recipients with a Cochlear™ Nucleus® 7 Sound Processor to complete a series of hearing tests from their compatible iOS device using the Nucleus Smart App.* Results are then sent remotely to the recipient’s clinic for review by their clinician. The clinician can review the results by logging into their myCochlear Clinic professional portal, where they can access a comprehensive overview of their patient’s hearing health. Remote Check is designed to provide a snapshot of a patient’s hearing health so a clinician can quickly determine whether a patient is progressing well, or whether further clinical intervention is required.**
“The FDA’s expedited approval of our Remote Check offering during the COVID-19 crisis underscores that at-home hearing healthcare support needs to be prioritized, more accessible and convenient for patients now more than ever,” said Patricia Trautwein, Au.D., Vice President, Marketing & Product Management, Cochlear Americas. “When elective surgeries become available again after the COVID-19 crisis, clinicians are going to see a wave of patients coming in to seek hearing implant treatment. Having a solution like Remote Check will help clinicians prioritize their case load, so they can reduce unnecessary visits for patients progressing well, spend more quality time with patients who have complex needs, and help more patients seeking initial cochlear implant candidacy assessment.”
For the Cochlear recipient, Remote Check is designed to reduce unnecessary travel burdens to get into their clinic, provide immediate hearing and patient data to their clinician for troubleshooting requests, and provides a convenient, time-saving option for care that does not require travel to the clinic. Using this technology, clinicians will not only be able to provide a more convenient avenue of care, but they can also free up more appointment times to manage the anticipated growth in the hearing implant market.
“Our clinic is eager to provide our patients with more remote servicing opportunities, and we are excited to start using Cochlear’s Remote Check offering,” said Regina Presley, Au.D., FAAA, CCC-A, Senior Cochlear Implant Audiologist, Presbyterian Board of Governors Cochlear Implant Center of Excellence, Greater Baltimore Medical Center. “Many patients travel long distances for in-clinic care, need a caregiver to help get to their appointments, have competing school, personal and professional priorities, so if we can provide at-home service options, we believe they will have a more positive healthcare experience overall. Every patient deserves timely, quality service to get the most from their hearing devices. Remote Check will ensure that patients receive the best care no matter where life and times like this take us.”
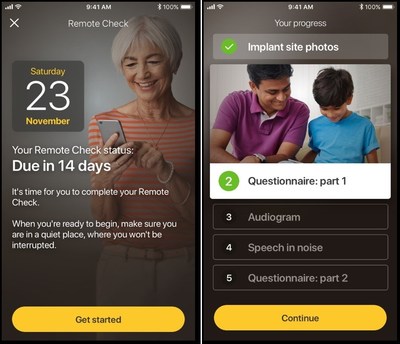
A clinician can choose to assign any of the following activities for their patient to complete as part of their Remote Check:
- Photographing the implant site and the area behind the ear.
- Completing questionnaires to gather information about general health and hearing.
- Taking an audiogram test to precisely measure the softest levels heard across a range of frequencies.
- Taking a listening-in-noise test to objectively assess hearing ability by identifying three-digit numbers presented in varying levels of background noise.
- Impedance Check to assess the performance of the implant.
The full suite of Remote Check activities for patients is designed to be completed in as little as 15 minutes, although it may take longer for some patients and children.1 Remote Check is designed so that it can be completed at a patient’s own pace. Early pilots of Remote Check in the United Kingdom and New Zealand affirm the need for the service with 87 percent of users stating they were likely to use Remote Check again.2
“Over the last decade, Cochlear has been heavily focused on expanding and improving our digital and remote care services. We’ve brought to market first-of-its-kind solutions, like Remote Check, remote programming, myCochlear Clinic, Cochlear Link and SignHEAR, to help improve clinic efficiency, support a range of clinical practice preferences and provide the best hearing experience for our recipients,” said Tony Manna, President, Cochlear Americas. “During unprecedented times like we’re experiencing right now, we’re proud Cochlear is the leader in providing innovative solutions that allow our clinic partners to continue treating their patients without the need for an in-person appointment, ultimately ensuring our recipients continue to hear and stay connected.”
About Cochlear Limited (ASX: COH)
Cochlear is the global leader in implantable hearing solutions. The company has a global workforce of more than 4,000 people and invests more than AUD$180 million each year in research and development. Products include cochlear implants, bone conduction implants and acoustic implants, which healthcare professionals use to treat a range of moderate to profound types of hearing loss.
Since 1981, Cochlear has provided more than 600,000 implantable devices, helping people of all ages, in more than 180 countries, to hear.
References
1. Based on internal Cochlear data. Some users may take longer than 15 minutes and 10 minutes, respectively. Data on file.
2. Based on internal Cochlear data. “Remote Check Pilot Evaluation UK & NZ.” Data on file.
* To use Remote Check, a patient requires: latest version of the Nucleus Smart App (Remote Check is part of this App) running on a compatible Apple device (iPhone or iPod Touch); Nucleus 7 Sound Processors with implant models: CI24RE, CI512, CI513, CI522, CI532, CI612, CI622 and CI632; compatible firmware on the Nucleus 7 Sound Processor (4.1.3.3 or later). A clinician requires access to Cochlear’s secure web-based myCochlear.com Professional Portal to access and review patient results. The recommended browser for accessing the Professional Portal is Google Chrome. A clinician also requires Custom Sound 5.1 or later software to upgrade the patient’s firmware to enable access to Remote Check. For sound processor and app compatibility information, visit www.cochlear.com/compatibility.
** Remote Check does not replace clinical care and does not involve remote programming of the sound processor.
Apple, iPhone and iPod touch are trademarks of Apple Inc., registered in the U.S. and other countries.
Please seek advice from your health professional about treatments for hearing loss. Outcomes may vary, and your health professional will advise you about the factors which could affect your outcome. Always read the instructions for use. Not all products are available in all countries. Please contact your local Cochlear representative for product information. Views expressed are those of the individual. Consult your health professional to determine if you are a candidate for Cochlear technology.
© Cochlear Limited 2020. All rights reserved. Hear now. And always and other trademarks and registered trademarks are the property of Cochlear Limited or Cochlear Bone Anchored Solutions AB.
View original content to download multimedia:http://www.prnewswire.com/news-releases/fda-expedites-approval-of-cochlears-remote-check-solution-for-cochlear-implants-301036357.html
SOURCE Cochlear Limited


